当前位置: Language Tips> 双语新闻
|
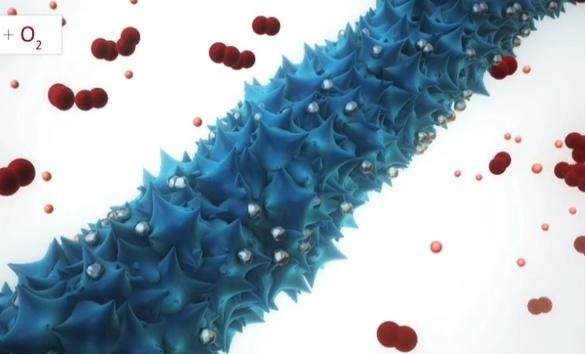
MIT researchers have found a way to boost lithium-air battery performance, with the help of modified viruses. Lithium-air batteries have become a hot research area in recent years: They hold the promise of drastically increasing power per battery weight, which could lead, for example, to electric cars with a much greater driving range. But bringing that promise to reality has faced a number of challenges, including the need to develop better, more durable materials for the batteries' electrodes and improving the number of charging-discharging cycles the batteries can withstand. Now, MIT researchers have found that adding genetically modified viruses to the production of nanowires -- wires that are about the width of a red blood cell, and which can serve as one of a battery's electrodes -- could help solve some of these problems. The new work is described in a paper published in the journal Nature Communications, co-authored by graduate student Dahyun Oh, professors Angela Belcher and Yang Shao-Horn, and three others. The key to their work was to increase the surface area of the wire, thus increasing the area where electrochemical activity takes place during charging or discharging of the battery. The researchers produced an array of nanowires, each about 80 nanometers across, using a genetically modified virus called M13, which can capture molecules of metals from water and bind them into structural shapes. In this case, wires of manganese oxide -- a "favorite material" for a lithium-air battery's cathode, Belcher says -- were actually made by the viruses. But unlike wires "grown" through conventional chemical methods, these virus-built nanowires have a rough, spiky surface, which dramatically increases their surface area. Belcher, the W.M. Keck Professor of Energy and an affiliate of MIT's Koch Institute for Integrative Cancer Research, explains that this process of biosynthesis is "really similar to how an abalone grows its shell" -- in that case, by collecting calcium from seawater and depositing it into a solid, linked structure. The increase in surface area produced by this method can provide "a big advantage," Belcher says, in lithium-air batteries' rate of charging and discharging. But the process also has other potential advantages, she says: Unlike conventional fabrication methods, which involve energy-intensive high temperatures and hazardous chemicals, this process can be carried out at room temperature using a water-based process. Also, rather than isolated wires, the viruses naturally produce a three-dimensional structure of cross-linked wires, which provides greater stability for an electrode. A final part of the process is the addition of a small amount of a metal, such as palladium, which greatly increases the electrical conductivity of the nanowires and allows them to catalyze reactions that take place during charging and discharging. Other groups have tried to produce such batteries using pure or highly concentrated metals as the electrodes, but this new process drastically lowers how much of the expensive material is needed. Altogether, these modifications have the potential to produce a battery that could provide two to three times greater energy density -- the amount of energy that can be stored for a given weight -- than today's best lithium-ion batteries, a closely related technology that is today's top contender, the researchers say. Belcher emphasizes that this is early-stage research, and much more work is needed to produce a lithium-air battery that's viable for commercial production. This work only looked at the production of one component, the cathode; other essential parts, including the electrolyte -- the ion conductor that lithium ions traverse from one of the battery's electrodes to the other -- require further research to find reliable, durable materials. Also, while this material was successfully tested through 50 cycles of charging and discharging, for practical use a battery must be capable of withstanding thousands of these cycles. While these experiments used viruses for the molecular assembly, Belcher says that once the best materials for such batteries are found and tested, actual manufacturing might be done in a different way. This has happened with past materials developed in her lab, she says: The chemistry was initially developed using biological methods, but then alternative means that were more easily scalable for industrial-scale production were substituted in the actual manufacturing. In addition to Oh, Belcher, and Shao-Horn, the work was carried out by MIT research scientists Jifa Qi and Yong Zhang and postdoc Yi-Chun Lu. The work was supported by the U.S. Army Research Office and the National Science Foundation. |
据外媒11月13日报道,麻省理工学院(MIT)研究人员发现,转基因病毒可以大大提升电池的性能。 锂空气电池近年来一直是研究热点,它的容电量有潜力大幅提高。不过,科学家需要先找到更加耐用的电极材料,增加电池的可充电次数,这个想法才能实现。 麻省理工学院研究人员让转基因病毒参与到纳米线的生产中,解决了一些技术难题。纳米线的宽度类似红细胞,在电池中可以用作电极。技术的关键在于增加纳米线的表面积,增加充电和用电过程中电极的活跃范围。这一研究成果已经在学术杂志《自然通讯》上发表。 一种名叫M13的转基因病毒能够抓取水中的金属分子,组成稳定的结构。科学家用M13病毒收集制作电池阴极的极佳材料——氧化锰,制造大约80纳米宽的氧化锰纳米线。和传统化学方法制造的纳米线相比,病毒纳米线表面粗糙不平,表面积显著增大。最后还要加入少量钯等金属元素,提升电极的导电性,促进充电和放电时的化学反应。 麻省理工学院能源教授贝尔彻说,M13的生物合成过程和鲍鱼长壳差不多。鲍鱼就是从海水中收集钙,再组成坚固的外壳。 有了病毒的帮助,新型电池的能量密度能够达到目前顶尖锂离子电池的2到3倍,并且在诸多方面优势明显。新电池电极表面积更大,充电和放电效率更高。其制造工艺更加简单、安全,病毒于常温状态下就能在水中完成工作,传统方法必须的高温条件和危险化学品已经没有用武之地。病毒制造的纳米线相互交错关联,制成的电极更加稳定。另外,电池对电极的金属材质要求降低,成本也更加合理。 贝尔彻强调说,研究还处于早期阶段,只做出了阴极,而电解液等关键部分仍然有待开发。此外,新型电池经测试可以充电50次,要真正应用,这个数字得上千才行。 贝尔彻还表示,目前在实验中虽然利用生物技术,通过病毒收集金属分子,但可能不是长久之计。如果将来找到最合适的材料,并且通过测试,工业生产中可能会采用别的办法,方便定量控制。 多年来,科学家一直热衷于病毒电池的研究。2010年,马里兰大学科学家让烟草花叶病毒(TMV)帮助电池进行化学反应,收集电流,增强电池的储电能力。同年,麻省理工学院科学家马克•艾伦也提出,可以利用M13病毒制造氟化铁阴极,希望制造轻巧持久的可充电池。 相关阅读 (王琦琛 编辑:信莲) |
上一篇 : 缩小贫富差距是当务之急
下一篇 : 查尔斯年满65岁 尚未接班就领退休金
关注和订阅

电话:8610-84883645
传真:8610-84883500
Email: languagetips@chinadaily.com.cn